This article was originally published in the January 2020 issue of AAOS Now, the American Academy of Orthopaedic Surgeons (AAOS) monthly news magazine. The article has been updated to reflect current events.
The new registry for orthopaedic oncology data has moved toward the full implementation stage, following a successful year-long trial of the Musculoskeletal Tumor Registry (MsTR), which has wrapped up.
MsTR, an American Academy of Orthopaedic Surgeons (AAOS) Registry with support from the Musculoskeletal Tumor Society (MSTS), has joined the Academy’s growing portfolio of quality-improvement registries, which consists of the American Joint Replacement Registry (AJRR) and the Shoulder and Elbow Registry, along with the American Spine Registry (ASR), a collaboration with the American Association of Neurological Surgeons.
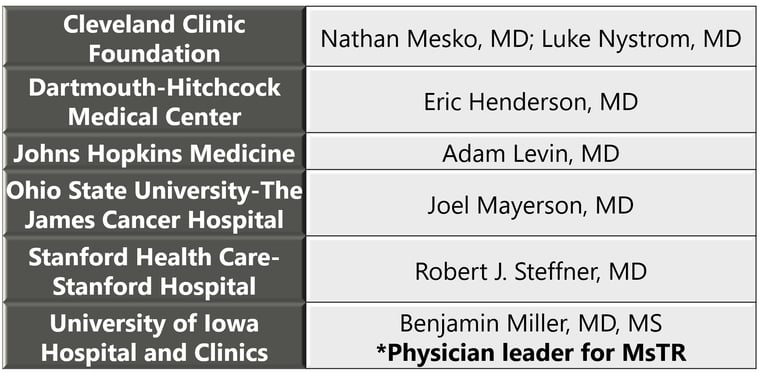
The wider rollout of the MsTR builds on experience developed with the six pilot sites and key surgeon leads that participated in 2019:
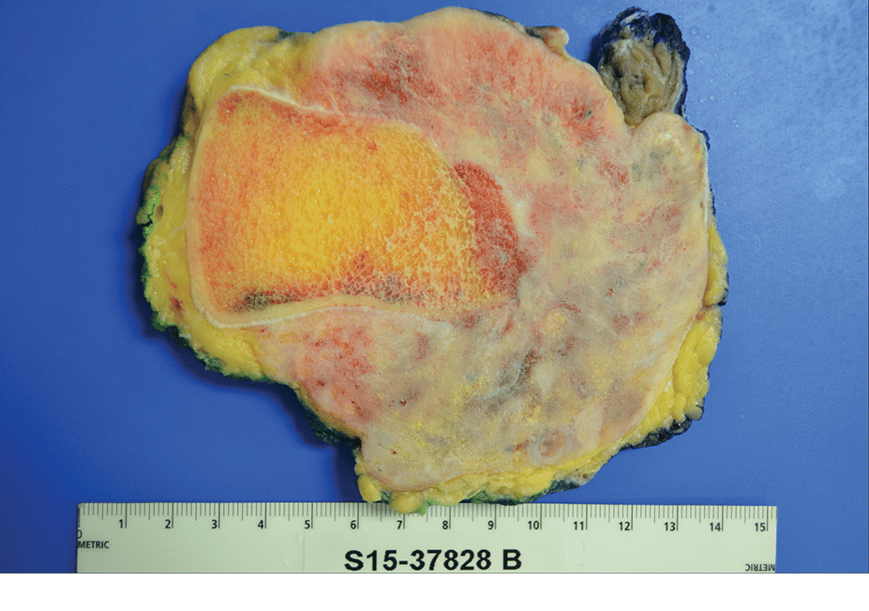
Those sites were charged with using individual institutional resources and workflows to determine the most efficient, simplest, and accurate methods to capture and submit data. Among the data elements tracked were patient demographics, patient baseline and examination, tumor baseline, treatment and post-treatment, and surgery detail, along with postoperative data (oncologic failure, surgery complications, vital status) and patient-reported outcomes (PROs), as conveyed in applicable instruments.
R. Lor Randall, MD, FACS, of the University of California, Davis, and president of MSTS at the time of the pilot launch, said then, “We are delighted to see this Herculean effort come to fruition. These patients are afflicted with rare and often aggressive tumors. Via this registry pilot, we will learn a great deal about how they are doing. Accordingly, we can then improve treatments and better address their needs.”
Benjamin Miller, MD, FAAOS, of the University of Iowa, said, “If the community of North American orthopaedic oncologists, through the MSTS, is able to collaborate through this Registry, we should be able to determine which interventions are most successful. This would help standardize treatments. In addition, patient counseling will become more accurate for oncologic results, reconstruction durability, functional outcomes, and long-term quality of life.”
Joel L. Mayerson, MD, of the Ohio State Comprehensive Cancer Center and current president of MSTS, added, “The Registry will allow surgeons to combine data about rare bone and soft-tissue tumors from institutions around the country, thereby potentially answering treatment and outcome questions that are otherwise unable to be answered due to the rarity of the disease process.” Nathan W. Mesko, MD, of Cleveland Clinic, noted, “This is the first initiative of collaboration on a national scale to obtain high-yield data in a reliable manner, answering questions with appropriate statistical power that can drive future care initiatives. It highlights the power of cooperation and partnership across institutional lines.”
The implementation of the full-fledged MsTR has drawn interest from centers across the country, Dr. Miller said. “MsTR might not seem so large in the context of AJRR, but most orthopaedic oncologists are at large regional referral centers and academic sites, so these sites are actually a substantial number representing a majority of sarcoma cases in the United States. When these interested sites follow the lead of the six pilot institutions and begin enrolling patients, the numbers included in the MsTR will be sizable enough to change how we take care of sarcoma patients,” he said.
The Tumor Challenge
In the MsTR, the planners and pilot site representatives faced the challenge of selecting appropriate data points for orthopaedic oncology.
“A tumor registry is a little different from the joint replacement or shoulder and elbow registries because it is diagnosis-based versus procedure-based,” Dr. Miller explained. “In the other two, you can define a procedure like total knee arthroplasty or rotator cuff repair as the mechanism to simply and silently identify patients for inclusion.”
“With tumor, it’s a little more complex,” he continued. “Our question involves recording the results and complications starting with the initial treatment of a pelvic or extremity sarcoma. A patient may carry a diagnosis of sarcoma, but if it is recurrent, or they are presenting for management of sequelae of the disease or treatment, then they would not qualify for the Registry. Further, treatment of sarcoma can include many procedures, none of which is absolutely unique to sarcoma. For instance, excision of a thigh mass could mean excision of a malignancy, benign tumor, or non-neoplastic condition.”
These factors add complexity to the task of data collection, Dr. Miller said. “As a group, we had to agree on the important data points, in particular our outcome measures of interest. Our outcomes include the common cancer endpoints of overall survival and local recurrence, but also PROs of function, pain relief, and quality of life.”
The primary goal of the 2019 MsTR trial program, he said, was to solidify the data elements and the means by which they are entered. “Our goal is to have the data entered with ease, so clinicians may capture registry data in seconds, while concurrently documenting the patient encounter in the medical record,” he said.
Opening for Business
The process of making the MsTR fully operational has now begun as the platform has opened its doors to new participants.
Interest has been keen, Dr. Miller said. At the October 2019 meeting of the MSTS, where he and colleagues gave a progress report on the MsTR pilot, Dr. Miller said, “It was encouraging and rewarding to stand up and say the pilot trial is reaching a successful conclusion, and we are ready to take the next step.”
He said all centers with orthopaedic oncology programs are encouraged to participate: “The tenets of this endeavor were to collect research-quality data, to make the burden of information input as minimal as possible for the [professional], and to be inclusive.”
“Sarcoma is a rare disease,” Dr. Miller continued. “More observations will lead to more results and data-driven conclusions that will change our practice for the better. This registry will allow for feedback to contributing [professionals] and centers through dashboards and periodic comparisons to national and personal quality and patient safety benchmarks that will be helpful not just to the clinicians but to their health systems. Registry participation is increasingly viewed as an option, perhaps an expectation, to be considered a high-quality institution. We believe that orthopaedic oncologists can make the case that participation in the registry is as good for the institution as it is for the surgeons themselves. This will be a fee-based service to ensure sustainability and functionality over time; however, MSTS and AAOS agree that it is important to be as inclusive as possible. Any and all sarcoma centers interested in participating are encouraged to contact the MsTR team at the AAOS Registry Program to gather more information and start the enrollment process.”
“Clinical research and advancements in orthopaedic oncology have historically been hindered by limited numbers, heterogeneous disease presentations, and varied treatments. The MsTR has potential to provide insight into oncologic and functional outcomes for many sarcoma subtypes and surgical procedures on a scale not previously possible,” Dr. Miller concluded.
Individuals or organizations interested in learning more about the MsTR can visit www.aaos.org/registries and may direct inquires to RegistryInfo@aaos.org.
Terry Stanton is the senior science writer for AAOS Now. He can be reached at tstanton@aaos.org.
Be sure to leave a comment in the form below!


