This article was originally published in the December 2018 issue of AAOS Now, the American Academy of Orthopaedic Surgeons' (AAOS) monthly news magazine.
The National Evaluation System for health Technology Coordinating Center (NESTcc), an initiative of the Medical Device Innovation Consortium (MDIC), announced the first round of NESTcc test-cases that address topics of high priority from the medical device industry using real-world data and evidence.
“It’s exciting to reveal this milestone for NEST with the launch of these first test-cases… with health systems and coordinated registry networks through the NESTcc network collaborators,” said Rachael Fleurence, PhD, executive director of NESTcc.
The initial concepts were submitted in January 2018 through a public call open to medical device manufacturers. The test-cases will address two primary objectives. First, they will explore the feasibility for the medical device industry to work with real-world data sources and NESTcc’s initial set of network collaborators. Second, the test-cases will help identify areas where NESTcc could play a role in reducing transaction costs (e.g., contracting, institutional review board, data-sharing agreements, publication policies).
The first round of test-cases will be executed through collaborations with industry partners Abbott, Adhesys Medical, Johnson & Johnson Medical Devices Companies, and W.L. Gore & Associates, Inc. In addition to the industry groups working through independent collaborations, AAOS is serving as a neutral convener, representing DJO Global, DePuy Synthes, Smith & Nephew, Stryker, and Zimmer Biomet for a test-case that will bring together NESTcc network collaborators with the American Joint Replacement Registry (AJRR), which is part of AAOS.
“AAOS’ collaboration with NESTcc demonstrates our continued commitment to improve the safety and quality of care for orthopaedic patients,” said AAOS President David A. Halsey, MD. “AJRR is an excellent resource to help determine more accurate device performance, survivorship, and surgical outcomes for total joint arthroplasty. This effort supports standardized care and quality improvement.”
Paul Voorhorst, MS, MBA, vice president of clinical research at DePuy Synthes, said, “The funding commitment by NESTcc to support the feasibility study of linking data from the AJRR with data from private payers is a testament to multistakeholder collaborations. It is exciting to think about where these types of collaborations will lead us and how patients will ultimately benefit through enhanced surveillance methodologies.”
The industry partners and network collaborators are working together on eight projects within the first round of test-cases, which include projects along the 510(k) and premarket approval regulatory pathways and throughout the medical device total product life cycle.
“This is an important step to show the potential for NEST to efficiently generate evidence for both medical device evaluation and post-market safety information,” said Jeff Shuren, MD, director of the Center for Devices and Radiological Health at the Food and Drug Administration and a board member of MDIC.
“Our goal with NEST is to improve access to, value, use, and efficiency of real-world evidence to meet the needs of the medical device ecosystem stakeholders. With more robust, informative, and timely evidence, health care providers and patients can make better-informed health care decisions and be assured they have access to the safest medical devices,” he added.
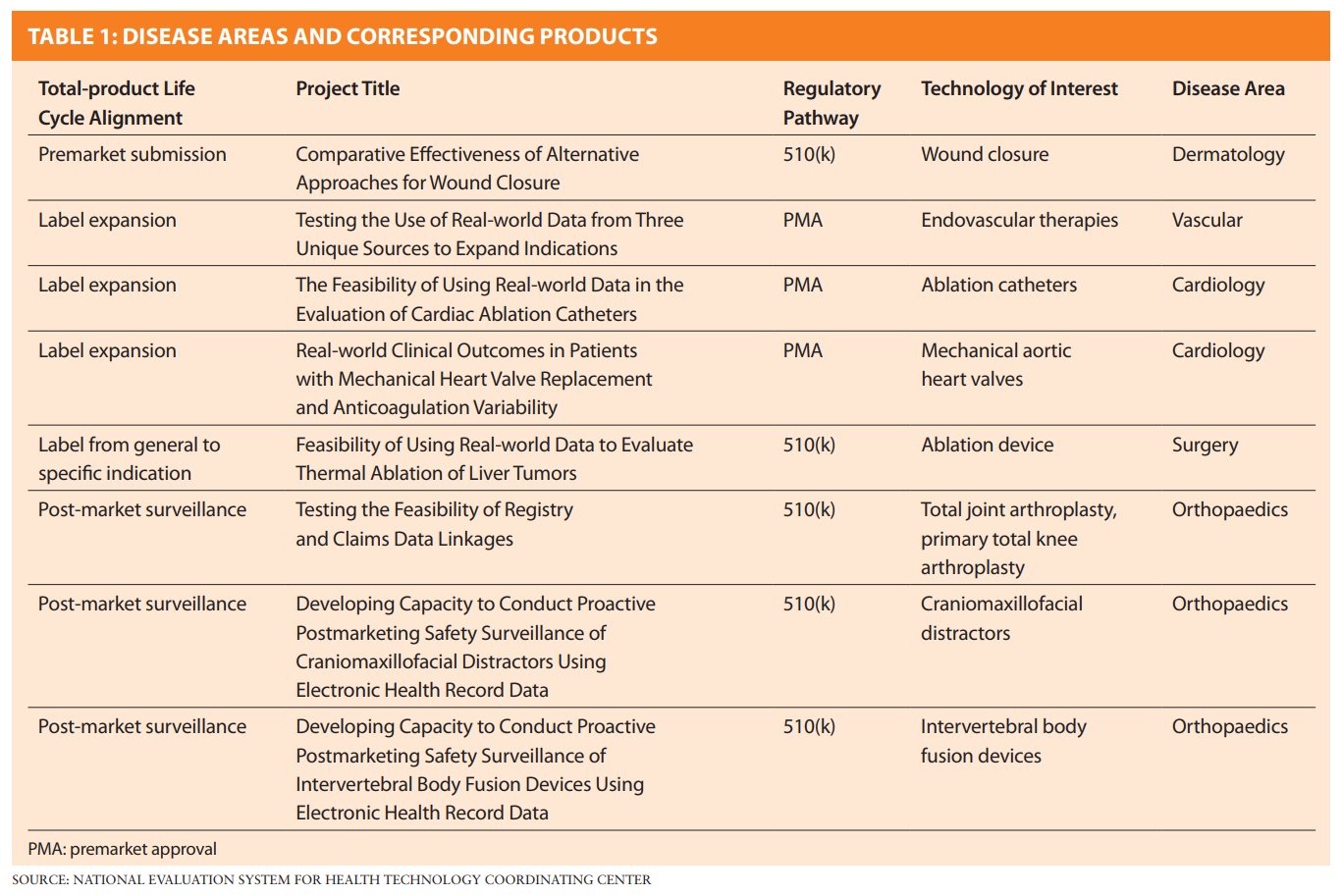
The test-cases will address specific disease areas and corresponding products (see Table 1 below). AJRR is assisting with the sixth Total-product Life Cycle Alignment item from the top.
For a description of each test-case, visit https://nestcc.org/test-cases.
Read more about this NESTcc initiative in their November 5, 2018, press release here.
For information about the AAOS RegistryInsights™ platform, speak with a Registry Engagement Associate at (847) 292-0530 or Request A Demo today!
Be sure to leave a comment in the form below!