The American Academy of Orthopaedic Surgeons (AAOS) RegistryInsights™ provides users with an overview of their submitted procedures and of the registry as a whole. From the homepage, users can navigate to the American Joint Replacement Registry (AJRR) and new Shoulder & Elbow Registry, the musculoskeletal tumor pilot, and other offerings as they become available.
This article was originally published in the December 2018 issue of AAOS Now, the AAOS' monthly news magazine.
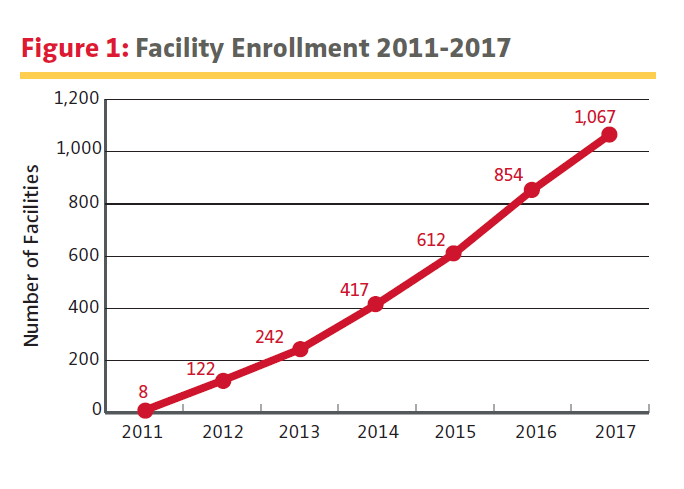
It’s been a year of milestones and trailblazing accomplishments for the AAOS Registry Program. In its 2018 Annual Report, having completed its reintegration within AAOS, the AJRR tallied 1,186,955 procedures from 1,067 institutions from 2012 to 2017, establishing the AJRR as the largest orthopaedic registry by annual procedure count.
"Registries are tools to help the medical profession collect data, report, and benchmark to define patient-centered quality care to identify potential procedural or implant problems before they become widespread public health issues," said AAOS President David A. Halsey, MD. "Other national joint replacement registries such as the Swedish Hip Arthroplasty Register have proven that monitoring survivorship can reduce revision rates. That translates into reducing direct medical costs and, more importantly, improving patient care and quality of life."
Hip and Knee
"It has been an eventful year for AJRR," wrote Kevin J. Bozic, MD, MBA, chair of the AJRR Steering Committee, in the AJRR 2018 Annual Report introduction. "New subscriber growth, innovative initiatives, and collaborations (none more significant than rejoining AAOS) have all contributed to the momentum the Registry experienced in 2017. The information in this year’s Annual Report gives the most comprehensive picture to date of patterns of hip and knee arthroplasty use and outcomes in the United States. The report contains analysis of new data elements and data sources this year, which led to new sections in the report to include an overview of data completeness, patient-reported outcome measures, and implant survivorship curves. With the rapid growth of AJRR capabilities, we look forward to being able to provide all our stakeholders valuable data that can be used to change practice and improve patient outcomes."
Furthermore, the AJRR 2018 Annual Report reflects data from the former California Joint Replacement Registry, which is now fully integrated into AJRR as the California State Registry. Read more about AJRR’s new relationship with The Joint Commission (TJC) in TJC section below.
Shoulder and Elbow
Standing out among accomplishments on the Academy Registry front was the launch of the Shoulder & Elbow Registry (SER) in October. Currently, SER collects data on shoulder arthroplasty procedures; in 2019, it will be expanded to capture data on rotator cuff repairs and elbow arthroplasty procedures.
Shoulder and elbow were identified and prioritized by the AAOS Registry Oversight Committee (ROC) as the next clinical area for a new registry, explained Daniel J. Berry, MD, chair of the committee. "These are high-volume procedures that are performed across the specialty," Dr. Berry said. "It became clear that shoulder and elbow needed a high-quality registry. Many AJRR clients already perform shoulder arthroplasty. They will be able to enroll in this module and easily add to their contracts, as well as recruit additional participants."
"SER, the first of a number of planned offerings emerging from the experience of highly successful AJRR, demonstrates the Academy’s commitment to providing the infrastructure for our members to study outcomes of their procedures," said Gerald R. Williams Jr., MD, chair of the SER Steering Committee. "As a member-driven organization, we offer this as value to our members, to help the frontline orthopaedic surgeon performing these procedures collect quality data without having to create the entire infrastructure him- or herself."
He noted that the Registry was developed with the support of organizational allies - namely, the American Shoulder and Elbow Society (ASES), American Orthopaedic Society for Sports Medicine, Arthroscopy Association of North America, and American Society for Surgery of the Hand - as will be future additions to the Registry roster covering other areas of focus. "We appreciate the help and support of our specialty societies for each anatomic Registry," Dr. Williams said.
"In 1917, American shoulder surgery pioneer Ernest Amory Codman challenged that patients should be followed long enough to determine if treatments proved successful. More than 100 years later, AAOS and ASES are taking a huge step toward that goal," said Grant E. Garrigues, MD, a shoulder and elbow surgeon. "Every ASES member must take up this charge and truly follow our shoulder patients’ outcomes, pool this data with other surgeons, and determine the optimal treatments for our patients. This Registry is a powerful tool to make this possible. This type of data registry is the key to improving patient outcomes and defining best practices."
Dr. Williams explained that a registry for shoulder and elbow procedures follows logically from the inaugural AJRR enterprise covering hip and knee replacement. "One of the reason shoulder and elbow was chosen was that people thought it was a good bridge to the mostly inpatient setting of hip and knee," he said. "Shoulder replacements are moving to a stronger outpatient emphasis in comparison to hip and knee. Most rotator cuff repairs are done in the outpatient setting. SER builds on the AJRR registry structure to include outpatient collection of data, which will help make a smooth transition for future registries."
A Tumor Pilot
This year, the Academy’s Registry Program also initiated a pilot project, in collaboration with the Musculoskeletal Tumor Society, a musculoskeletal tumor registry pilot for data covering procedures in othropaedic oncology. The effort involves six pilot sites at major U.S. academic centers.
Data elements for the tumor Registry will include procedural records - covering demographic, patient baseline and examination, tumor baseline, treatment and post-treatment, and surgery details - as well as postoperative data (oncologic failure, surgery complication, vital status) and patient-reported outcomes as conveyed in applicable instruments.
A Joint Effort - With The Joint Commission
The role of registries in general, and the AJRR in particular, gained further official recognition in September with the announcement that The Joint Commission would implement a new advanced certification provision for total hip and knee replacement (THKR), requiring hospitals and ambulatory surgery centers (ASCs) to participate in a national registry to further help standardize care and quality improvement in hip and knee replacements. The AJRR will become the sole pathway for meeting The Joint Commission’s THKR advanced certification registry participation requirement effective July 1, 2019.
Awarded for a two-year period, the THKR designation is a voluntary advanced certification established by The Joint Commission in 2016 for accredited hospitals, critical access hospitals, and ASCs, providing them with a framework and pathway for improving patient outcomes that now will include participation in the AJRR.
"This collaboration with The Joint Commission, and the THKR program, is a practical way for us to even better equip orthopaedic surgeons to continue to advance the quality of musculoskeletal care," Dr. Halsey said.
"Organizations that achieve The Joint Commission’s already rigorous THKR Certification will gain an even stronger foundation for ensuring highly reliable high-quality care and outcomes for their patients through the Academy’s collaboration with our program," said David Baker, MD, executive vice president in the Division of Healthcare Quality Evaluation at The Joint Commission.
Other Strides
The Academy’s registry initiatives recorded progress and accomplishments in several other ways, including:
- Governance. Now that the AJRR has been fully integrated into the Academy's overall registry initiative, "the Registry Oversight Committee has greatly benefitted from the lessons learned and insights provided by the AJRR," Dr. Berry said. "This wealth of information was useful in the development and implementation of SER." The ROC also recently announced that its Public Advisory Board, which had been serving just the AJRR, will now serve all registries, with a member added for SER to represent the patient and public perspective.
- Product improvements. A number of enhancements and new capabilities have been added to the RegistryInsights™ platform to improve functionality and optimize the user experience. By the end of the year, the platform will be revamped, and in 2019, new and improved modules - including surgeon dashboards - will be available, with improved transparency into data submission status.
- Data linkage. AJRR was the first Qualified Clinical Data Registry to obtain Centers for Medicare & Medicaid Services claims data from the Research Data Assistance Center process to facilitate linkages to its data, including more complete comorbidity information and knowledge of revisions performed in non-AJRR sites, with the goal of providing this information back to participants throughout the year. "The theme is to decrease the burden and increase the value," Dr. Berry said.
- A market channel for data. The Registry Program has implemented new policies and procedures for using Registry data for peer-reviewed abstracts and manuscripts. The first manuscript submissions occurred, and three abstracts were accepted for presentation at the AAOS Annual Meeting in March 2019. Additionally, the increased exposure of Registry data into the market included two presentations at the American Association of Hip and Knee Surgeon's annual meeting in November 2018 and four at the International Society of Arthroplasty Registries conference in July 2018.
- NESTcc. In November, the National Evaluation System for health Technology Coordinating Center (NESTcc), an initiative of the Medical Device Innovation Consortium, announced the first round of test-cases in priority topics for the medical device industry using real-world evidence and data. Within this effort, in addition to industry groups working through independent collaborations, AAOS serves as a neutral convener, bringing together orthopaedic industry companies for a test case that join NESTcc network collaborators with the AJRR. "AAOS' collaboration with NESTcc demonstrates our continued commitment to improve the safety and quality of care for orthopaedic patients," said Dr. Halsey. "AJRR is an excellent resource to help determine more accurate device performance, survivorship, and surgical outcomes for total joint arthroplasty. This effort supports standardized care and quality improvement."
- MOC inclusion. At press time, the Academy was informed by the American Board of Orthopaedic Surgery that the AAOS Registry Program was approved as an alternate choice for earning self-assessment examination credits for the Maintenance of Certification (MOC) process. The details of how to use surgeon-level access and reports for MOC-qualifying improvement activities and how to claim credit will be released in early 2019.
Participants Welcome
Academy members interested in becoming part of the registry effort—including as physician champions for their practice site—are encouraged to contact the Academy Registry Program, at RegistryInfo@aaos.org. "We have a dedicated team to help them through the contracting and support the onboarding process," Dr. Berry said. "For surgeons who want to get involved, our staff is available to help their teams make it happen."
Terry Stanton is the senior science writer for AAOS Now. He can be reached at tstanton@aaos.org.
For information about the AAOS RegistryInsights™ platform, speak with a Registry Engagement Associate at (847) 292-0530 or Request A Demo today!
Be sure to leave a comment in the form below!